What is Developmental Biology?
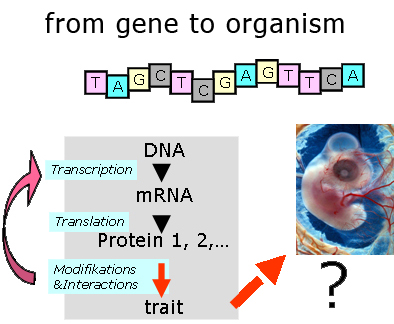
Developmental Biology analyses highly integrated feedback processes at molecular, cellular and histological levels, which direct the entire development from fertilisation of the egg through maturation of the adult organism. As systems of study, we use appropriate model embryos, e.g. from chicken, zebrafish or mouse.
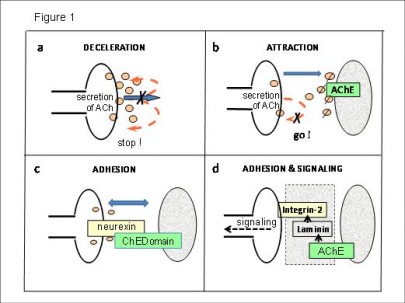
This knowledge is ground-breaking for all biomedical progress (e.g. stem cell biology, regenerative medicine, cancer research), as well as for a better understanding of evolution. In this group, we investigate molecular and cellular processes of development of the eyes (retina), the brain, or limbs. Thereby, acetylcholine and its protein components (e.g. cholinesterases) play a central role.
What is Neurogenetics?
Neurogenetics as part of developmental biology investigates molecular bases of the embryonic formation of nervous systems. A general question is, how a huge number of specific neuronal connections as required for a functional brain (1016/person) can be established, given that the total number of genes in man is very limited (2x104/person). Thereby, we analyse roles of acetylcholine and various growth factors.
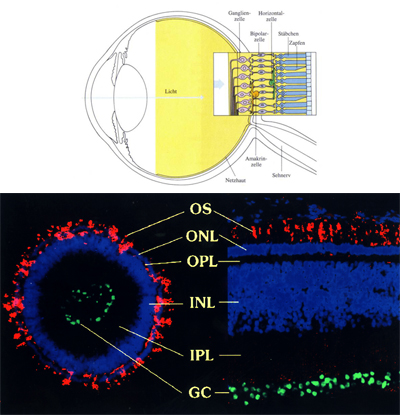
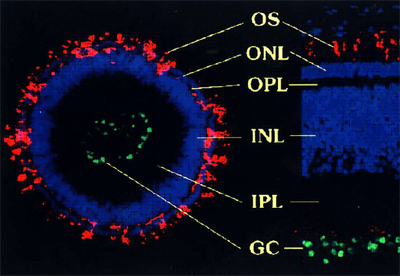
Due to its simple layered structure, we focus on the development of the vertebrate retina in eyes from chicken and mouse embryos. Our cell biological techniques also allow us to culture artificial retinal tissues from stem cells (e.g. retinal spheroids), as well as develop methods for replacement of animal experiments.